This nanomedicine project is led by Lars Herfindal. He took his PhD in cell biology at the Department of Biomedicine, University of Bergen in 2006, with emphasis on novel therapeutic agents against acute myeloid leukaemia (AML). He has received grants from Helse-Vest and the Norwegian Cancer Society to improve chemotherapy against cancer by using nanocarriers. Herfindal has been trained in nanoparticle production at the laboratory of Dr. Gillian Barratt at Institut Galien, University of Paris (11-sud).
Treatment of cancer using cytostatics faces several challenges, which make the therapy inefficient and sometimes also toxic to the patient. Not only will most of the drug be distributed at other places than the exact location of the cancer, but most of it will be excreted or metabolised in the body before it have time to act on the cancer cells. Also, most, if not all cytostatics have unwanted side-effects on normal tissue, ranging from rather innocent ones like hair loss or mild skin problems, to more severe and sometimes fatal consequences like loss of proper intestinal function, bone marrow depletion or heart failure. Although the use of several different cytostatics in combination have led to milder and more efficient therapies, the clinicians still face the dilemma where the side-effects of the cytostatic therapy is more severe than the disease itself.
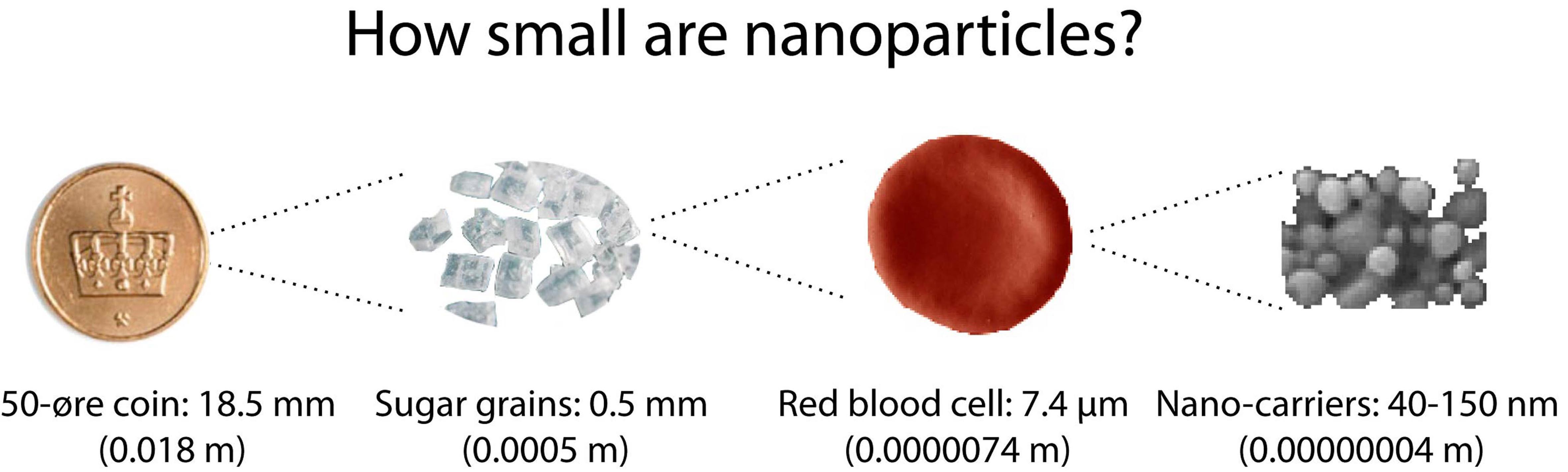
An attractive way to improve cancer therapy by cytostatics is the use of dedicated drug carriers. Such carriers have size of 20-200 nm, small enough that they can circulate freely in the blood. They are often referred to as nano-carriers, or nanoparticles.
If you cover a small coin with one layer of nanocarriers, you need about 20 trillion, or 20 000 000 000 000 nanocarriers.
Our aim is to use such nano-carriers to improve treatment of cancer which cannot be removed surgically, such as leukaemias and solid cancers at a metastatic state.
1: Improved drug loading in solid tumours.
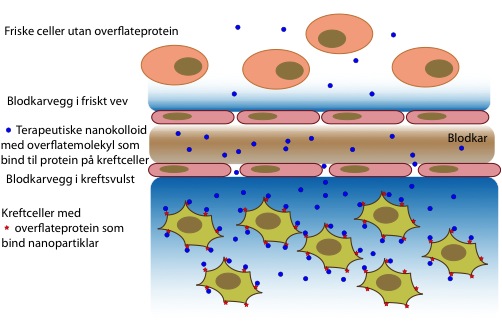
One of the main problems with chemotherapy is that it usually affects the whole body, rather than only the malignant cells. Nano-carriers have been used to improve this, since they can keep the drug away from the normal tissue, and also since they tend to accumulate in solid tumours. The latter is due to the “enhanced permeability and retention” (EPR) effect seen in tumours. The blood vessels in tumours have clefts that allows small particles to diffuse through the blood vessels passively. Moreover, there is a lack of lymphatic drainage, which usually removes excess fluid from tissues. However, this net influx in one direction causes a high pressure in the tumours, wich can prevent drugs and drug carriers to evade from the blood and enter the interstitial space where the cancer cells grow.
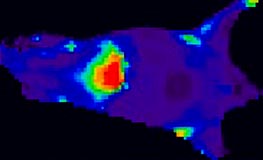
We will try to enhance the EPR effect further. To obtain this, we will manipulate two key elements in the tumour: First, we will increase the space between the cells in the blood vessels. This will ensure that more nano-carriers can diffuse into the interstitial tissue. Secondly, we will reduce the interstitial pressure by relaxing the cells that makes up most of this tissue. Nanoparticle accumulation will be measured by in vivo imaging of tumour-bearing mice.
Distribution of fluorescent nanoparticles in a mouse. The more red, the higher concentration of nanoparticles. Note the high accumulation in the liver, which is typical for almost all nanoparticles unless they are coated for “stealth” effect.
One must remember that most of the cells in a tumour are in fact not cancer cells, but normal cells that the tumour cells have recruited to make a favourable environment for growth. To eradicate the tumour, it is natural to also manipulate these normal cells. We hope to be able to prime the tumour so the response by nano-sized chemotherapy will be increased.
The mechanisms that can cause accumulation of nanoparticles in tumour tissue (below blood vessel) compared to normal tissue (above blood vessel).
2: Nanocarriers to treat acute myeloid leukaemia
Leukaemia is a difficult cancer to deal with, since it cannot be surgically removed. New advances in stem cell therapy or transplantation therapy has improved survival in many patients, but all leukaemia patients will undergo chemotherapy of some kind. However, chemotherapy of leukaemia faces the same challenges as with other cancers.
Besides unwanted side-effects, a common problem is relapse of the leukaemia, which usually results in a more aggressive disease that is more difficult to eradicate. To improve the toxicity towards leukaemia cells, we will label the nanocarriers with molecules that binds to protein on the surface of the cells. There are several such proteins that are only expressed on cancer cells, and a recently started proteomic project (led by Prof. Bruserud) can reveal surface proteins that are present on chemoresistant cells from for instance patients with relapsed leukaemia.
We have already showed improved efficacy of a liposomal drug carrier coated with folate on the surface to target to leukaemia cells. Now, we aim to enhance leukaemia cell selectivity using antibody-coated liposomes. In addition, our search for natural compounds with anti-leukaemic activity has yielded us with some very interesting results. However, these compounds have low solubility and poor pharmacokinetics. This can be improved by encapsulation in nano-carriers. One of the compounds will be encapsulated in cyclodextrins, which again can be trapped inside liposomes, to ensure targeted delivery to leukaemia cells.
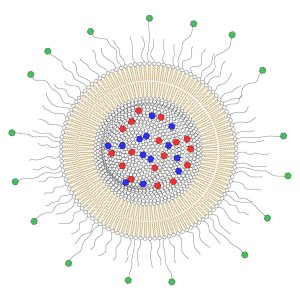
Example of a liposomal drug carrier for targeted delivery. The lumen is filled with a cocktail of drugs (red and blue), the membrane ensures that the drugs are kept inside the carrier. The outer surface is covered with polymer chains to prevent degradation of the nanocarrier by immune cells, and some polymers have molecules that attach on surface proteins on cancer cells only (green).
New molecules to treat leukemia
Despite the emerging advances in cell based therapies, treatment of cancer will rely heavily on small molecules therapeutics.